The Emergence of CROs in Drug Development
New drug development is a complex process that requires enormous time and cost. Each development stage—including clinical trials—demands specialized expertise and personnel, and there are practical limits to performing everything in‑house. Against this backdrop, CROs (Contract Research Organizations), known in Korean as “임상시험수탁기관,” emerged. CROs are specialized service firms that enable pharmaceutical and biotech companies to outsource clinical trial and R&D activities. Since the 1970s, the CRO industry has become a core pillar of the drug development ecosystem over roughly four decades. Unlike the past—when drug makers carried out most tasks themselves—today most companies receive CRO support from clinical trial planning through operations. In fact, one survey reported that 97% of respondents outsource some portion of clinical trial planning or operations to CROs, underscoring that CRO use has become a mainstream strategy in drug development.
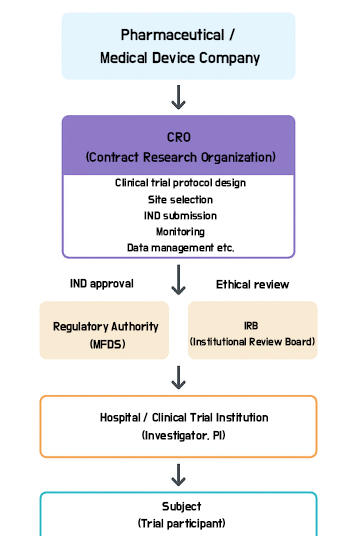
Figure 1. Example infographic illustrating the concept of a CRO. A CRO designs and manages clinical trials on behalf of the sponsor (the pharmaceutical company) and collaborates with clinical trial sites (hospitals) to conduct the study. As shown, CROs serve as a bridge between the sponsor and the clinical trial site in new drug development. While they do not usually interact directly with patients, they support hospital research staff and oversee the overall trial process.
Definition and Key Roles of CRO
A CRO is a specialized company contracted by pharmaceutical companies, biotechs, and medical device developers to conduct clinical trials and various research tasks, providing a broad range of services per contract. CROs support the entire clinical development continuum, including protocol drafting, study design, subject recruitment and management, data management and biostatistics, monitoring and quality management, and interactions with regulators. Typical CRO services include:
Clinical trial preparation: Develop study plans; draft essential documents such as the protocol and informed consent form (ICF); manage submissions and obtain approvals from regulators (e.g., submitting a clinical trial application to the MFDS in Korea).
Site and investigator selection: Identify suitable clinical trial sites (hospitals) and principal investigators for the therapeutic area; organize investigator meetings.
Ethics review and contracting: Prepare Institutional Review Board (IRB) submission packages for each hospital; support execution of site contracts.
Clinical monitoring: Conduct on‑site monitoring with CRAs; track enrollment; verify protocol compliance; collect and manage data.
Data management and analysis: Build clinical trial databases; validate data integrity; plan and perform statistical analyses.
Reporting and regulatory support: Draft clinical study reports (CSRs); report outcomes to authorities such as the MFDS; support preparation of marketing authorization/approval applications.
Pharmacovigilance (PV): Collect and report adverse events during trials; perform post‑marketing surveillance (PMS) and other safety activities.
As a partner responsible for overall trial operations, a CRO performs specified tasks at the sponsor’s request. However, regulations emphasize that the sponsor retains ultimate responsibility for trial ethics and data integrity; even when outsourcing to a CRO, the sponsor must manage and oversee outcomes. Depending on scope, CROs may handle Phase I through Phase III and post‑marketing studies, while some focus on specialized areas such as preclinical research, sample analysis, or biostatistics. By size and capability, offerings range from full‑service CROs to specialty CROs, and sponsors select the most suitable partner based on project needs.
Growth of the Global CRO Industry
Fueled by the broader outsourcing trend in pharma/biotech R&D, the CRO industry has grown rapidly worldwide. As pressure mounts to reduce development costs and shorten timelines, companies minimize internal investment in personnel and facilities by leveraging specialized contractors for clinical trials. Consequently, the global CRO market has expanded sharply year after year.
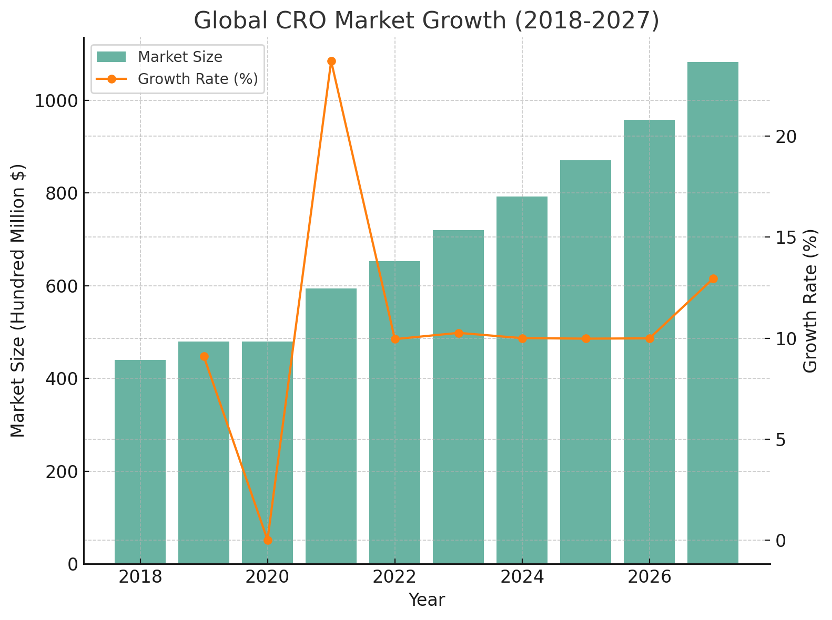
Figure 2. Global CRO market size (bars, US$ hundred millions) and annual growth rate (line, %) from 2018 to 2027. In 2021, the global CRO market reached approximately US$59.39 billion, up 24.3% year‑over‑year. Sustaining a double‑digit CAGR above 10%, it is projected to reach about US$108.2 billion by 2027. The COVID‑19 pandemic increased trial demand and accelerated vaccine/therapeutic development, further propelling CRO growth. As outsourcing intensifies across the continuum—from preclinical to late‑stage trials—both large pharma and smaller biotechs view CRO engagement as essential. Global top‑10 CROs, including IQVIA, Labcorp, ICON, Parexel, and PPD, lead the market, with a substantial combined share.
Trends in the Korean CRO Industry
Mirroring global dynamics, Korea’s CRO sector has maintained steady growth. As of 2024, Korea conducts about 3.46% of sponsor‑initiated clinical trials worldwide, ranking 6th globally; notably, Seoul ranked #1 among cities for number of clinical trials conducted from 2017 to 2023. Backed by robust clinical infrastructure and research capability, multinational drug makers actively run clinical trials in Korea. Historically, many trials in Korea were led by global CROs alongside multinational pharma, but domestic CROs have grown rapidly as local new drug development increases and government support strengthens.
There are roughly 20–30 CRO companies operating domestically. By revenue, foreign‑affiliated CROs historically held an advantage with ~70% share. However, driven by double‑digit growth—domestic CROs recorded an average annual growth rate exceeding 11% over the past decade—2020 marked the first time total revenue of domestic CROs (KRW 284.4 billion) surpassed that of foreign CROs (KRW 269.8 billion), according to one study. Government initiatives—such as the establishment of KONECT and CRO accreditation—appear to have contributed. Even so, global CROs continue to command a significant portion of the Korean market based on their worldwide networks and deep experience. Observers note that domestic firms still need to further strengthen specialized talent and global project experience.
Korea’s clinical environment—characterized by top‑tier medical standards in Asia and rapid patient enrollment—has attracted international attention. Building on this, Korean pharma/biotech companies increasingly leverage CRO partnerships from early‑stage studies to global expansion trials. Even start‑ups unfamiliar with clinical procedures and regulations can navigate complex processes more smoothly with an appropriate CRO partner. Specialist firms such as Intoinworld share up‑to‑date information on Korean clinical procedures, regulatory compliance, and success cases, contributing to industry advancement. For details, see intoinworld’s clinical trials information page.
The Role of CROs and the Importance of Partnership
CROs are indispensable in the pharmaceutical and biotech industry. As competition in new drug development intensifies and innovative technologies emerge, partnering with a dependable CRO that possesses the right expertise is key to increasing success probabilities and saving time and money. By engaging CROs, sponsors can optimize internal resources and entrust complex trial operations to experienced professionals, allowing teams to focus on development strategy and core research. When selecting a CRO, sponsors should carefully evaluate therapeutic‑area experience, global networks, and regulatory capabilities, and maintain close communication to align on objectives and quality standards. Ultimately, collaborative partnership between sponsor and CRO is essential to deliver successful clinical trials.
Working with a credible, expert CRO helps manage the risks and complexities inherent in bringing new therapies to patients. Around the world, countless clinical trials proceed with CRO support, enabling domestic and international pharma/biotech companies to accelerate time‑to‑market for innovative medicines through strategic partnerships. Finding the right partner for your program is critical—consider active collaboration with a CRO to increase the odds of successful development.
If you are preparing for a clinical trial, consulting with a professional CRO can ensure tailored support for your project. If you have an innovative idea, don’t hesitate to seek expert guidance to raise your chances of clinical success.
Planning a new clinical trial project? Intoinworld is a leading Korean CRO with rich experience and expertise, ready to help partners execute trials successfully. Contact us now for consultation and guidance on CRO partnerships. And if you don’t want to miss the latest pharma/biotech industry trends and clinical trial insights, consider subscribing to Intoinworld’s newsletter to get valuable information on a regular basis.
FAQ
Q1. Do I have to use a CRO for every clinical trial?
A. Not necessarily. However, most pharmaceutical and biotech companies actively leverage CROs for efficiency and expertise. Clinical trials involve complex procedures and strict regulatory compliance, so companies without dedicated in‑house teams often benefit from partnering with a CRO in terms of time and cost. For large, multi‑country Phase III trials, experienced global CROs help reduce missteps and safeguard quality. Conversely, smaller early‑phase studies may be executed by internal teams; even then, partial outsourcing (e.g., monitoring or data management) is common to fill capability gaps.
Q2. What should I consider when selecting a CRO?
A. Evaluate therapeutic‑area experience, past success cases, specialized personnel and infrastructure, and regulatory capabilities. CROs with deep experience in your target indication have advantages in patient enrollment and study design. Review similar projects they have successfully delivered. Also assess the quality of their data management systems, biostatistics capabilities, financial stability, and communication. Finally, ensure contract terms (budget, timelines) are clear and that collaboration will be smooth, then determine overall fit as a long‑term partner.
Q3. How do global CROs differ from domestic (Korean) CROs?
A. Global CROs operate networks across many countries and can manage multi‑regional trials, with extensive experience in international studies and up‑to‑date knowledge of global regulations—advantages for large, multi‑country programs. Korea‑based (local) CROs know MFDS procedures and local hospital networks intimately, and communication is often smoother due to language/time‑zone alignment; they may also be more cost‑effective. Choose based on project needs: consider global CROs for trials aimed at worldwide approval and Korean CROs for domestic studies or those requiring nuanced local execution.