Korea’s Ministry of Food and Drug Safety (MFDS) implemented a comprehensive overhaul of its new drug approval and review system effective January 1, 2025. Key measures such as dedicated review teams, expanded face-to-face consultations, a new rolling review process, and parallel Good Manufacturing Practice (GMP) inspections aim to shorten the total approval timeline from about 420 days to 295 days. This article outlines the core changes of the reform, analyzes their impact on clinical trials and new drug development, and discusses how the specialized expertise of a Korea-based contract research organization (CRO) like Intoinworld can assist sponsors under the new regulatory framework.
Background and Objectives of the Overhaul
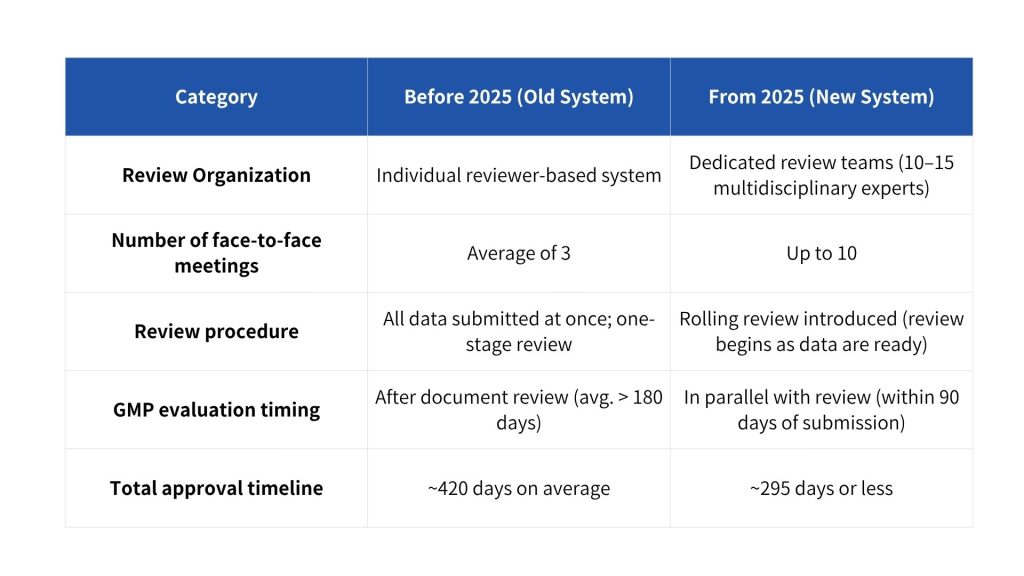
As of January 1, 2025, MFDS has radically overhauled its new drug approval and review framework. This initiative was driven by inefficiencies and lack of predictability in the previous system. In the past, reviews were handled by individual reviewers, which proved limiting when dealing with new therapeutic areas or complex data—one person simply could not manage every aspect. Official face-to-face consultation opportunities were limited to an average of only three meetings, making it difficult to resolve all issues in such short interactions. The review process often saw repeated requests for additional data and subsequent delays. Notably, GMP compliance inspections were conducted separately after the document review phase, taking on average over 180 days. As a result, the entire approval process typically took around 420 days (approximately 14 months). This lengthy and fragmented process slowed down new drug development and led to unexpected cost increases for pharmaceutical companies.
The goal of the MFDS overhaul is to improve consistency and efficiency in reviews, thereby shortening the overall time to approval and establishing a transparent, predictable process. The new system targets completing the process from new drug application submission to approval in 295 days or less. To achieve this, several key measures have been introduced, including the formation of dedicated review teams for each application, an increase in the number of sponsor–reviewer meetings, the introduction of a “rolling review” submission process, and conducting GMP inspections in parallel with document review. To support this innovative process, MFDS has dramatically increased the new drug application fee to approximately KRW 410 million (from about KRW 8.83 million previously) in order to secure additional personnel and strengthen review capabilities. By deploying more specialized reviewers funded by this higher fee, MFDS intends to provide faster yet expert reviews. In essence, the overhaul is aimed at enhancing transparency and predictability in the review process to support faster market entry for new drugs.
Key Changes in the New Review System
Introduction of Dedicated Review Teams – Strengthening Consistency
Under the new system, once a new drug application is filed, a dedicated review team is assembled for that product. Each team consists of roughly 10–15 experts from diverse fields (e.g. pharmacology/toxicology, clinical, quality, regulatory), who collectively handle the application from the initial consultation stage through final approval. No longer is the review outcome dependent on the perspective of a single reviewer; with specialists in each domain working in parallel, evaluations have become more comprehensive and consistent. The dedicated team begins full-scale review within two weeks of application submission, and the same members remain involved throughout the process. This continuity minimizes the inconsistencies or duplicate inquiries that can arise from reviewer handoffs. MFDS has stated that this shift to an integrated, product-focused team review structure will improve both the quality and efficiency of reviews. In fact, the agency has significantly expanded its pool of expert reviewers—adding over 30 new reviewers dedicated to new drug teams—to bolster its review capacity. For pharmaceutical companies, the operation of a dedicated review team means they now have a consistent point of contact for communications and a unified review process that maintains context from the initial meeting to final decision.
Expanded Consultations & Rolling Review Procedure – Enhanced Communication and Real-Time Review
Throughout the new drug review process, formal face-to-face meetings between MFDS reviewers and the sponsoring company (applicant) have been greatly expanded. Previously, a sponsor would officially meet with MFDS reviewers only about three times on average during the review, but now they can have up to 10 or more in-person meetings as needed. If issues arise during the review, the two sides can now coordinate in real time through more frequent meetings. In particular, when reviewers request supplemental data, communication is no longer limited to official documents and paperwork (which used to cause significant delays). Now, from the point of a data request to the submission of a response, unlimited face-to-face meetings can be held to clarify and address the issues. In practice, since the overhaul, whenever a sponsor submits supplementary materials, the dedicated team of 15–17 members convenes for over an hour to discuss the data. The sponsor is invited to explain the prepared responses in person, resolving the reviewers’ concerns on the spot. This proactive communication increases transparency in the review process and reduces misunderstandings or additional delays.
In addition, MFDS has introduced a new “rolling review” procedure. This system allows the review to commence sequentially with available sections of the dossier, rather than waiting until all application materials are ready. For example, if quality (CMC) data or certain nonclinical/clinical study results are available earlier, the sponsor can submit those parts first, and the review team will begin evaluating those sections while the remaining data are being prepared. By using rolling review, sponsors can reduce idle time spent waiting for the complete data package to be finalized, thereby moving up the overall approval timeline. Notably, even while final clinical trial data are being compiled after study completion, the already-submitted portions of the dossier can be under review concurrently—shortening the time to ultimate approval. This process change essentially regularizes the “rolling” or continuous review concept that was employed for emergency use authorizations (such as COVID-19 vaccines), and it has brought a significant shift in how companies strategize their regulatory submissions.
Parallel GMP Evaluation & Shorter Approval Timeline – Targeting Approval Within 295 Days
Perhaps the most attention-grabbing change is the drastic reduction in the total approval timeline. Under the new review system, MFDS conducts GMP compliance evaluation in parallel with the document review, with the goal of shortening the overall approval timeline from an average of 420 days to within 295 days. To enable this, the GMP inspection process was reformed to run concurrently with the review of the application. In the past, a GMP inspection of the manufacturing site took place only after the dossier review was completed, resulting in considerable additional delay. Now, the schedule is set so that GMP evaluation and on-site inspections are completed within 90 days of the new drug application submission. Each review team includes a GMP specialist who participates alongside the rest of the team, conducting the product review and GMP review simultaneously. This parallel approach avoids duplicate data submissions and accelerates the inspection schedule.
Along with this, MFDS has overhauled several related administrative procedures to streamline GMP evaluation. For instance, when registering an imported active pharmaceutical ingredient (API), instead of requiring a separate local factory inspection, the process can now accept a foreign GMP certificate that meets WHO/PIC/S standards. This change has slashed that particular step from about 120 days to just 20 days. Additionally, the number of GMP-related documents required at the time of new drug application has been consolidated from 11 items to 4, reducing the documentation burden. Periodic GMP re-inspections of manufacturing sites can now be replaced with desk audits (paper-based assessments) if the risk is deemed low. All of these changes are aimed at eliminating inefficiencies during the approval process and allowing reviewers to focus on the substantive evaluation.
As a result of implementing these innovative review measures, the average time to approval for new drugs in Korea has been shortened from roughly 14 months to about 10 months. This review speed is now among the fastest globally, which is expected to accelerate patient access to new therapies and speed up market entry for companies. Indeed, MFDS announced that by establishing dedicated teams and conducting document review and GMP inspections in parallel, it has cut the approval timeline from 420 days to 295 days, emphasizing that it is operating a review system that is professional, rapid, transparent, and predictable. In the period since the overhaul took effect in early 2025, more than 10 new drug applications have already been processed under the new framework. Some global pharmaceutical products are anticipated to receive approval as early as the end of this year, providing tangible evidence of the reform’s impact.
Impact on Clinical Trials and New Drug Development
This MFDS regulatory overhaul carries significant implications for the pharmaceutical and biotechnology industry, both in Korea and internationally. The most direct impact is the shortening of project timelines for new drug development. By trimming over four months off the regulatory review stage, the market launch of new drugs can occur that much sooner. Because new drugs have limited patent terms and market exclusivity periods, the timing of their launch has a substantial effect on a company’s revenue.
According to an analysis by the Seoul Economic Daily, gaining approval just one month earlier can generate enough additional sales to offset the increased application fee; a four-month acceleration can therefore yield major economic benefits for a sponsor company. Ultimately, patients also benefit by gaining faster access to innovative treatments, a clear positive from a public health perspective.
At the same time, the changes in the review process require pharmaceutical companies to adjust their preparation and response strategies. First, to effectively work with a dedicated review team and more frequent meetings, companies need to strengthen their internal regulatory affairs capabilities. To discuss issues efficiently across as many as 10 meetings, it has become important to prepare an agenda and data package tailored to each meeting and to communicate strategically. In particular, cross-functional collaboration—among clinical, quality, and regulatory affairs departments—is essential so that potential issues are anticipated and solutions are developed in advance.
With the introduction of the rolling review, managing the completeness and consistency of submitted data has become even more crucial. In a parallel review setup, clinical trial data integrity is scrutinized under stricter standards, so companies must have quality management systems in place to ensure consistency across submission modules and to track any changes. In short, companies should revamp their regulatory submission strategies and exercise thorough project management throughout the entire process, from the pre-submission consultation stage to the completion of the review.
Meanwhile, the hefty increase in the new drug application fee could pose a financial burden for small and mid-sized pharma or startups. However, by using the higher fees to expand its pool of expert reviewers and enhance review rigor, MFDS is expected to actually lower the risk of approval failure or extensive delays. Moreover, early trends after the overhaul show that despite the fee hike, the number of new drug applications has remained on par with previous years, indicating that the industry is generally eager to take advantage of the new process.
The reform is also seen as a positive development for foreign pharmaceutical companies seeking to enter the Korean market. With greater transparency and predictability in the review process, challenges related to language barriers or lack of local regulatory information are mitigated, reducing the perceived regulatory risk of entering Korea. That said, foreign firms will still need to continuously monitor the ever-changing regulatory landscape and communicate smoothly with the Korean authorities—a task that can be demanding. In this regard, partnering with a local CRO or regulatory consulting firm familiar with the Korean environment can be immensely helpful.
For example, getting expert partner support—from generating the necessary clinical trial results and preparing documentation, to handling MFDS pre-submission meetings and responses to deficiencies—can save significant time and resources. In fact, when global pharmaceutical companies conduct clinical trials and seek approvals in Korea, they often collaborate with local CROs to gain up-to-date regulatory information and to have administrative procedures handled on their behalf. (Intoinworld’s blog offers a Clinical Trial Information section that provides useful updates on Korean clinical trial regulations and procedures.)
With this overhaul making every step from clinical development planning to approval application more complex, a strategy that is gaining attention is to work with a Korea-based CRO that has experienced professionals. Such partnerships can help companies reduce risks and increase their chances of success under the new system.
Intoinworld’s Expertise and Role
In a constantly evolving regulatory environment, devising and executing an accurate regulatory strategy is key to successful new drug development. Intoinworld has been fulfilling this role as a Korean CRO, helping pharmaceutical companies navigate the Korean market efficiently and reliably. In anticipation of the MFDS review system overhaul, Intoinworld has also been leveraging its extensive expertise to ensure that its clients can adapt smoothly to the new procedures.
Intoinworld Co., Ltd. has over a decade of experience providing full-service support to numerous domestic and global pharma/biotech companies, from clinical trials through regulatory approval. It offers one-stop services covering all steps of the process: from clinical trial design and MFDS pre-submission consultation preparation, to new drug application dossier writing and submission, managing responses to supplemental inquiries, and compiling final clinical study reports.
Each project is handled by teams of experts with rich field experience in their respective domains, who work together to proactively identify and address potential issues even under the revamped review procedures. For instance, Intoinworld provides finely-tuned support such as devising customized consultation strategies and data packages in line with the frequent meeting schedule of the dedicated review teams, and formulating stepwise document submission plans to prepare for rolling review. The team also continuously monitors the latest MFDS guidelines and regulatory trends and rapidly integrates those updates into clients’ development programs, minimizing unnecessary delays or rework and helping to secure approvals in the shortest time possible.
By collaborating with a specialized CRO like Intoinworld, companies that are encountering these complex approval procedures for the first time can more easily comply with Korean regulations while advancing their development. Intoinworld is in fact one of the few integrated full-service CROs in Korea, and it has used its wealth of experience in both drug and medical device trials to effectively eliminate language and administrative barriers for global clients entering Korea. Accurately understanding new regulatory systems and applying them to fit each client’s project is the very mission of Intoinworld, and through this expertise it provides solutions that achieve both speed and quality from the clinical trial phase all the way to final approval. In an era of rapid regulatory change, having a reliable partner can turn the uncertainty of change into confidence, enabling pharmaceutical companies to attain faster success in new drug development.
FAQ
Q1: What has changed with the MFDS’s new drug review system overhaul in 2025?
A: The biggest change is a complete innovation of the approval review process. For each application, a dedicated review team of about 10–15 members is now assembled to consistently handle the review from start to finish, and the number of official face-to-face consultation meetings between reviewers and the company has been increased to up to 10 sessions. Additionally, a “rolling review” procedure has been introduced, allowing the review to begin sequentially with available data instead of waiting for the full dossier, and GMP evaluations are conducted in parallel with the document review. Together, these changes — dedicated teams, enhanced communication, and concurrent reviews — have established a faster and more predictable approval system, cutting the overall new drug approval timeline from around 420 days to 295 days.
Q2: By how much has the review timeline accelerated, and why is this important?
A: Before the overhaul, new drug reviews in Korea took an average of about 14 months (around 420 days). After the overhaul, the target timeline has been shortened to roughly 10 months (within 295 days). An approval that is more than four months faster is extremely significant for pharmaceutical companies. Launching a drug earlier means that patients can receive innovative therapies sooner, and it also lengthens the period of market exclusivity, making it easier for the company to recoup its R&D investment. One analysis indicated that even a one-month acceleration in the approval timing can generate additional revenue well above the increased application fee. Therefore, a shorter review timeline positively impacts both patient treatment opportunities and the company’s bottom line.
Q3: How should pharmaceutical and biotech companies prepare for the changed system?
A: First, companies need to strengthen their regulatory strategy. In anticipation of working with dedicated review teams and more frequent meetings, it is important to organize a list of potential issues starting from the pre-submission consultation stage, and prepare the necessary data and Q&A responses for each meeting. Relevant departments such as clinical development, quality, and regulatory affairs should collaborate so they can respond swiftly to any requests for supplementary information — establishing an internal process to handle such inquiries.
In addition, since the rolling review allows for partial submissions, companies should continuously ensure that their clinical data and CMC documents are complete and maintain consistency across all modules. For overseas companies, partnering with a local Korean CRO to bridge information gaps regarding regulatory changes is also an effective approach. In practice, a specialized CRO can help prepare submission documents aligned with the latest MFDS requirements, and provide language and administrative support to guide the sponsor smoothly through the revised clinical trial and approval process. By taking these steps, companies can continue to manage their new drug development timelines proactively, even under the new review system.